QUALITY
GENERAL-BIOCHEMICAL
POLLEN (Ph. Eur. 2621 Pollen 01/2017:2627)
TEST PARAMETER (Limit)
- Macroscopic identification (Bot. Key)
- Microscopic identification (Bot. Key)
- Purity (>90%)
- Size of pollen (μm)
- Purity: Nº of grains examined (≥500)
- Spores (≤1%)
- Foreign pollen mix (≤1%)
- Other vegetal parts (≤10 %)
- Pesticide residues (Eu. Ph. 2.8.13)
- Heavy metals (Eu. Ph. 2.4.27)
- Residual solvent (Eu.Ph. 2.4.24)
- SDS-PAGE (IUIS compatibility)
- Water Content (≤ 7%)
- Total protein BCA
- Microbial contamination (UFC/gr)
- Storage Refrigerator (2-8ºC)
- Shipping Condition (Ambient temperature)

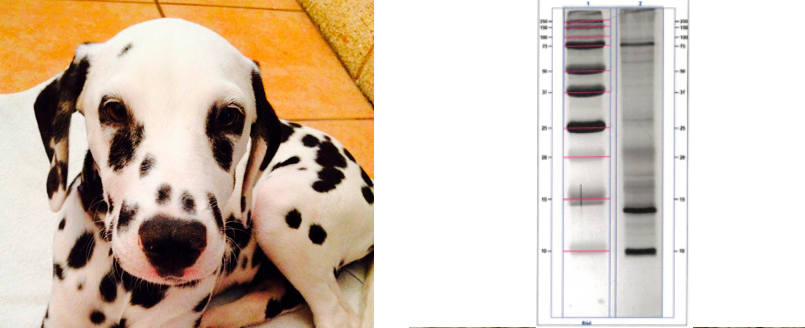
DANDER (Ph. Eur. 2621 Animal epithelia and outgrowths for allergen products)
TEST PARAMETER (Limit)
- Macroscopic identification
- Foreign matter (0%)
- Animal health certificate
- SDS-PAGE (IUIS compatibility)
- Water Content (≤ 7%)
- Total protein BCA
- Microbial contamination (UFC/gr)
- Storage Freezer (-15-30ºC)
-
Shipping Condition (Ambient temperature)
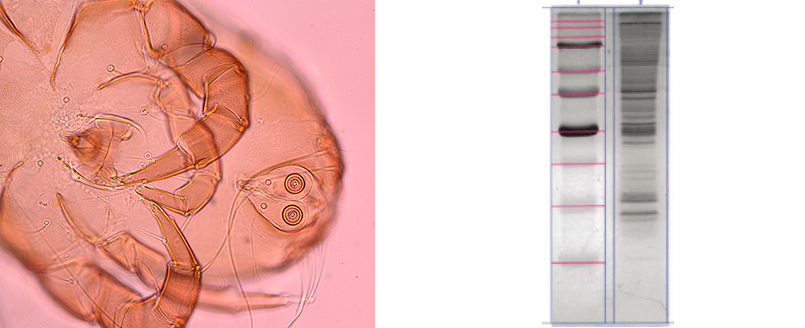
MITES (Ph. Eur. 2625 Mites for allergen products)
TEST PARAMETER (Limit)
- Microscopic identification (Int. Key)
- Mite bodies purity (≥90%)
- Foreign mites (0%)
- SDS-PAGE (IUIS compatibility)
- Water Content (frozen raw material)
- Total protein Bradford
- Microbial contamination (UFC/gr)
- Storage Freezer (-15-30ºC)
- Shipping Condition (-15-30ºC).
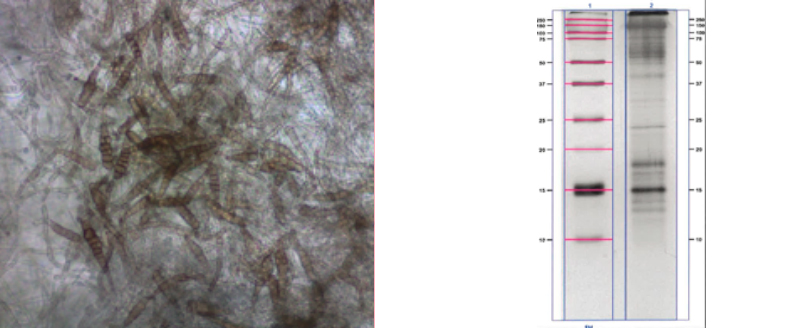
MOULDS (Ph. Eur. 2626 Moulds for allergen products)
TEST PARAMETER (Limit)
- Macroscopic identification (Int. Key)
- Microscopic identification (Int. Key)
- Partial Gene Sequencing (>99%)
- Purity (100%)
- Foreign moulds (0%)
- Microbial contamination (UFC/gr)
- Micotoxins (Absence)
- SDS-PAGE (IUIS compatibility)
- Water Content (≤ 7%)
- Total protein BCA
- Storage Freezer (-15-30º)
-
Shipping Condition (Ambient temperature)
Quality documents
Statement Danders
Please, contact our Customer Care Specialist
Statement of Culture Media
Please, contact our Customer Care Specialist